Description
UpCard-CA1 is a potent loop diuretic with a once daily administration to help manage pulmonary edema in dogs with congestive heart failure caused by myxomatous mitral valve disease (MMVD).
Indications
UpCard-CA1 is indicated for use with concurrent therapy with pimobendan, spironolactone, and an angiotensin converting enzyme (ACE) inhibitor for the management of pulmonary edema in dogs with congestive heart failure caused by MMVD. UpCard-CA1 is conditionally approved by the FDA pending a full demonstration of effectiveness under application number 141-577. It is a violation of Federal law to use this product other than as directed in the labeling.
Active Ingredient
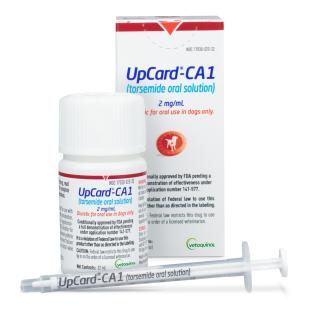
UpCard-CA1 is supplied at a concentration of 0.2% (2 mg) torsemide per mL.
Dosage and Administration
UpCard-CA1 should be administered at 0.05 to 0.2 mg/lb. (0.11 to 0.44 mg/kg) of bodyweight once daily.
UpCard-CA1 is available in an easily titrated oral solution for precise dosing adjustments compared to tablets.
Refer to the dosage and administration section of the full prescribing information for instructions.
How Packaged
UpCard-CA1 is available in a 32 mL bottle with a 1 mL syringe and a 96 mL bottle with a 10 mL syringe.
Where to Buy
UpCard-CA1 is available from a licensed veterinarian. Veterinarians can contact their Vetoquinol representative or preferred distributor.
Important Safety Information
UpCard-CA1 is conditionally approved by the FDA for use with concurrent therapy with pimobendan, spironolactone, and an angiotensin converting enzyme (ACE) inhibitor for the management of pulmonary edema in dogs with congestive heart failure caused by myxomatous mitral valve disease (MMVD).
IMPORTANT SAFETY INFORMATION:
UpCard-CA1 is for use in dogs only. Do not administer to dogs with renal failure, anuria, severe dehydration, hypovolemia, or hypotension. Do not administer UpCard-CA1 concomitantly with other loop diuretics or to dogs with hypersensitivity to the active substance, torsemide, or to any of the excipients. UpCard-CA1 should be used only in stable dogs with congestive heart failure caused by MMVD which has been diagnosed by means of a comprehensive physical and cardiac examination. This drug has not been evaluated in dogs used for breeding, pregnant or lactating bitches. The most common side effects seen in dogs with CHF due to MMVD while taking UpCard-CA1 are cough, dyspnea, pulmonary edema, and cardiac arrest. Adverse reactions not related to disease progression in dogs receiving UpCard-CA1 include polyuria and polydipsia, renal insufficiency, increased BUN and serum creatinine, urinary incontinence, hypokalemia, hypochloremia, hypercalcemia, hypomagnesemia, diarrhea, vomiting, and inappetence. For full prescribing information, visit www.vetoquinolusa.com/upcard-ca1-info.