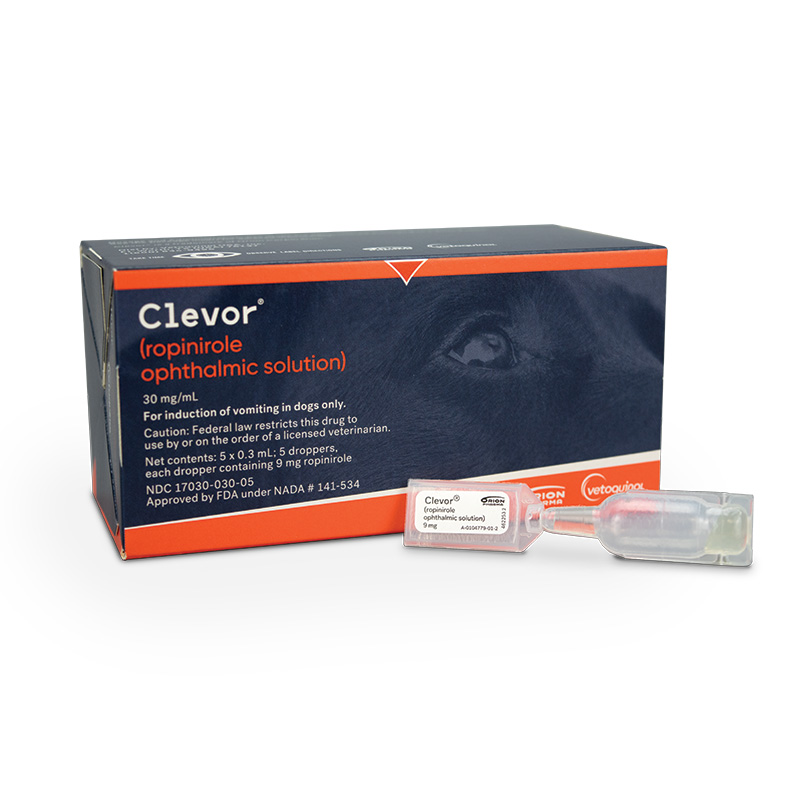
Clevor® (ropinirole ophthalmic solution) and ToxBuddy®, an online service to help quickly evaluate potential poisonings in dogs, are offered through Vetoquinol
FORT WORTH, TEXAS (April 15, 2021) – Vetoquinol USA announces the launch of two new offerings: Clevor®(ropinirole ophthalmic solution) and ToxBuddy®.
 Clevor is the first and only FDA-approved emetic for dogs, and ToxBuddy is an online information service that can help veterinarians quickly assess and treat potential canine poisonings.
Clevor is the first and only FDA-approved emetic for dogs, and ToxBuddy is an online information service that can help veterinarians quickly assess and treat potential canine poisonings.
“Cases where emesis is required are some of the most stressful situations for veterinarians and pet owners. It requires quick decision-making and reliable products,” said Eric M. Alsup, DVM, Country Manager, Vetoquinol USA. “We are proud to bring veterinarians Clevor, which is a selective emetic.1 Emesis isn’t required in all situations, which makes ToxBuddy a helpful service to streamline the management of these cases in the clinic.”
Clevor has a selective active ingredient, ropinirole, which quickly targets the right receptors for vomiting. Ropinirole is specifically selective for dopamine D2-type receptors, which cause emesis in dogs. Plus, Clevor is available in a convenient, ready-to-use dropper that provides one treatment for one dog.1,2
ToxBuddy is an online database that contains more than 300 substances that are toxic to dogs, including human medicines, household chemicals and plants. It provides a toxin calculator to help quickly assess dose and toxicity. ToxBuddy also offers guidelines for decontamination procedures, among other information. It is updated regularly with the latest guidance and can be accessed by the entire staff of the veterinary practice.
Both Clevor and ToxBuddy are owned by the Orion Corporation and are offered in the United States through a partnership with Vetoquinol.
CLEVOR® is indicated for the induction of vomiting in dogs.
IMPORTANT SAFETY INFORMATION: Do not use in dogs with central nervous system depression or seizures. Do not use in cases of ingestion of sharp foreign objects, corrosive agents (acids or alkalis), volatile substances or organic solvents. CLEVOR® should not be administered in cases with corneal ulceration, ocular irritation, or ocular injury. Do not use when there is a known sensitivity to ropinirole or the inactive ingredients. ADVERSE REACTIONS MAY INCLUDE: Transient mild or moderate hyperemia of the eye, ocular discharge, protrusion of the 3rd eyelid and blepharospasm, transient mild lethargy and increased heart rate. Not recommended for use in breeding, pregnant or lactating dogs. CLEVOR® has not been evaluated in dogs with heart or liver impairments or dogs younger than 4.5 months or less than 4 pounds. Dopamine antagonists, neuroleptics and other medicines with antiemetic properties may reduce the effectiveness of ropinirole. CLEVOR® should be administered by a veterinary professional. Gloves and protective eyewear should be worn when administering. Not for use in humans. Keep out of reach of children. For full prescribing information go to: https://www.vetoquinolusa.com/clevor-info.
About Orion
Orion is a globally operating Finnish pharmaceutical company – a builder of well-being. Orion develops, manufactures and markets human and veterinary pharmaceuticals and active pharmaceutical ingredients. The company is continuously developing new drugs and treatment methods. The core therapy areas of Orion's pharmaceutical R&D are neurological disorders, oncology and respiratory diseases for which Orion develops inhaled pulmonary medication. Orion's net sales in 2020 amounted to EUR 1.078 million and the company had about 3,300 employees at the end of the year. Orion's A and B shares are listed on Nasdaq Helsinki.
About Vetoquinol USA
Headquartered in Fort Worth, Texas, Vetoquinol USA is owned by Vetoquinol S.A., an independent, family-owned French pharmaceutical company founded in 1933. Dedicated exclusively to animal health, Vetoquinol USA is focused on the development, production and marketing of FDA, EPA, NASC and AAFCO-regulated pharmaceutical, nutritional and dermatological products for small and large animals.
Contact
Jennifer Ryan
(620) 388-3937
usa_news@vetoquinol.com
1. Suokko M, et al. Ropinirole eye drops induce vomiting effectively in dogs. Vet Rec. 2020 Mar 7;186(9):283.
2. Clevor® (ropinirole ophthalmic solution) Prescribing Information. Orion Corporation. 2020.
Clevor® and ToxBuddy® are registered trademarks of the Orion Corporation. Clevor is manufactured by the Orion Corporation and distributed by Vetoquinol USA under license from the Orion Corporation.
© 2021 Vetoquinol USA. All rights reserved. CVR-0012-PRESSR
Latest news

Vetoquinol, Expert Educators Join Forces to Create Online Veterinary Rehabilitation Courses
April 2025
FDA Approves Extended Expiration Date for Clevor® (ropinirole ophthalmic solution)
August 2024

Press Release: Fluorescent Light Energy Enhances Post Mastectomy Wound Healing in Dogs
August 2024
